
 .
.
The new lightest material in the world.
.
Since the Nobel Prize was awarded in 2010 to the British scientists who carried out the first experiments with graphene, many researchers have supported them by inventing new materials from graphene or graphite (one of the elements that make up graphene).
The idea is to manufacture materials in three dimensions, with good electrical conductivity and, above all, light, which is why it is increasingly common for a research team to announce the discovery of the “lightest material in the world”.
The last to win this title is the airbrush, developed by scientists in northern Germany based on porous carbon tubes. With a weight of just 0.2 milligrams per cubic centimeter, they say it is extremely resistant.
The material not only has good electrical conductivity but also repels water, and the researchers believe it could be very useful in making lighter batteries.
The finding, the result of joint work between Kiel University and the Hamburg University of Technology, was published in the journal Advanced Materials of the month of July.
.
The light materials race
In November US scientists presented this lightweight material made from nickel.
 Only six months have passed since a material made from a framework of hollow nickel metal tubes with a density of 0.9 milligrams per cubic centimeter was presented. So light that it can be placed on top of a dandelion without slightly altering its shape.
Only six months have passed since a material made from a framework of hollow nickel metal tubes with a density of 0.9 milligrams per cubic centimeter was presented. So light that it can be placed on top of a dandelion without slightly altering its shape.
But according to the creators of aerographite, it is up to four times lighter because it is made of carbon, an element with a much lower atomic mass than nickel, and because its creators managed to make tubes with “porous” walls.
“It is a very flexible and robust material, which we can compress and which returns to its original shape without destroying its electrical connectivity,” Matthias Mecklenburg from the University of Hamburg explained to BBCMundo.
.
evaporated zinc
To make the material, powdered zinc oxide was used, which was heated to a temperature of 900 degrees Celsius until it became crystals. With them, nanometric structures known as “terapods” were made, the filaments of the “network” that make up the base structure to make aerographite.
Molded into a “pill”-like shape, this structure was processed in a chemical steam reactor at 760 degrees Celsius. With this process the material was enriched with carbon, leaving the zinc covered by a very fine layer of graphite.
“This forms the network structure of aerographite. Simultaneously, hydrogen is introduced. This reacts with the oxygen in the zinc oxide and results in the emission of zinc vapor and gas,” explained Karl Schulte, professor at the University of Hamburg.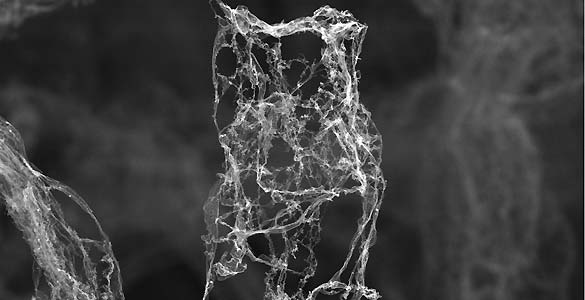
In this image taken by an electron microscope, the porous structures that make up the aerographite can be seen in detail.
It is through this process that the initial zinc structure is eliminated and only the porous cover remains.
Given its conductivity, the scientists believe that aerographite could be very useful in lithium-ion batteries, which could lead to a considerable reduction in their weight in electronic cars and electric bicycles, since, they said, its main contribution would be the development of greener vehicles.
However, the material could have many other applications, such as in electronic systems for aviation and satellites, as well as in water purification systems, since it could act as an absorbent for pollutants.
Source: BBC World


